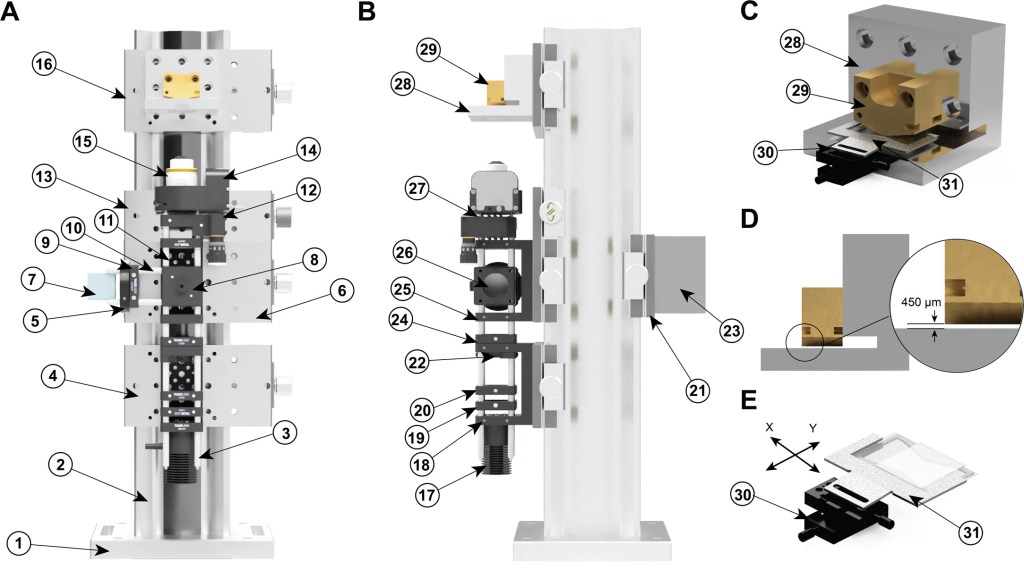
Single-Molecule Instrumentation
We develop single-molecule magnetic tweezers instrumentation, aiming at pushing our experimental boundaries, and also in an effort to disseminate this technology. Check our GitHub page for frequent updates on our magnetic tweezers designs and accompanying software

Mechanosensing Proteins
Cells need to sense and respond to mechanical forces imposed by their microenvironment. At the molecular level, such mechanosensing processes are underpinned by the activity of specialized proteins under force. Mechanosensing proteins—like talin, FAK, of paxillin—undergo conformational changes triggered by force, which develop into downstream signalling responses, namely force-dependent binding interactions. We employ high-resolution magnetic tweezers to measure the exquisite force response of these proteins and capture how mechanical forces regulate the recruitment of key ligand partners.
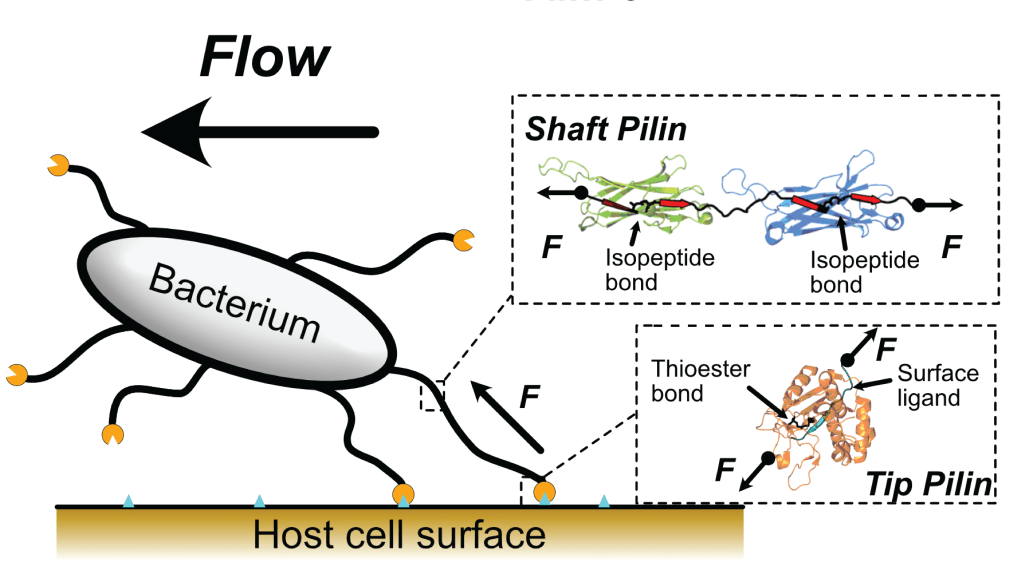
Mechanochemistry of Bacterial Adhesion
At the onset of an infection, bacteria adhere to host cells using their pili, which secure them against the formidable host defense mechanisms. Coughing, sneezing, or the mucus flow exert enormous mechanical forces aiming to detach the pilin anchors; however, bacterial pilin proteins have evolved smart chemical strategies that provide them with outstanding mechanical properties. We employ single-molecule techniques to unveil the chemical mechanisms underpinning pilin’s mechanical resilience, aiming at developing molecular strategies to interfere with bacterial adhesion.
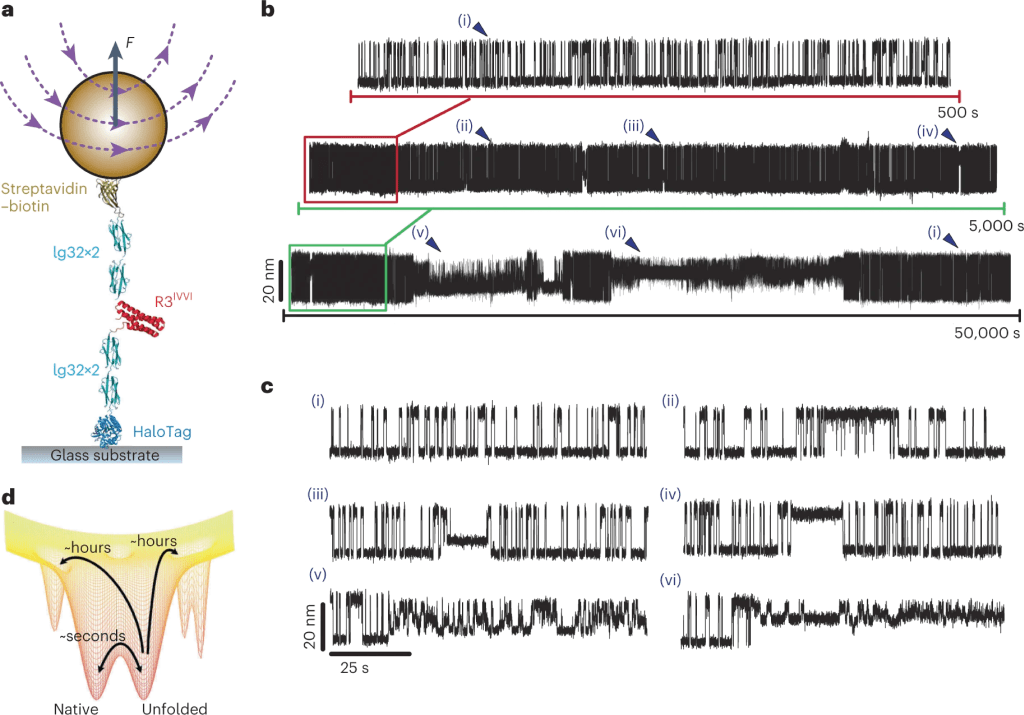
Protein Folding Dynamics
How proteins navigate their huge conformational space to reach and attain their folded, functional state remains one of the biggest mysteries in Biophysics. Recently, the classic static perception of protein structure has shifted towards a more dynamic view, where proteins can transit among several functional structures and, sometimes, fall into misfolded, kinetic traps, often underpinning diseases. Thanks to the outstanding stability of our magnetic tweezers approach, we aim to understand how proteins behave over long timescales, directly capturing the complexity of their landscapes.